Carbon dating is a widely-used scientific method for determining the age of organic materials that were once alive. This essay aims to explain the process of carbon dating, its limitations, and introduce other radiometric dating methods such as radiometric dating and cosmogenic nuclide dating.
Carbon Dating: Establishing Age through Radioactive Decay
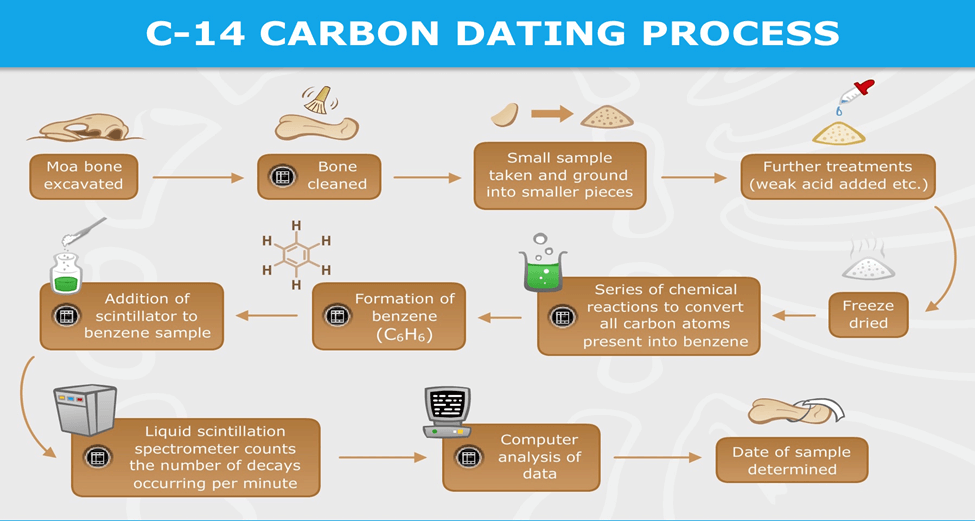
- Carbon-14 (C-14) and its Role:
- C-14 is an isotope of carbon with an atomic mass of 14 and is radioactive.
- Living organisms acquire carbon, including C-14, from the atmosphere through photosynthesis or food consumption.
- The ratio of C-12 to C-14 in the atmosphere is known and relatively stable.
- The Decay Process:
- When organisms die, they no longer interact with the atmosphere, and C-14 in their remains begins to decay.
- C-14 has a half-life of approximately 5,730 years, meaning it reduces to half its original amount over that time.
- By measuring the changing ratio of C-12 to C-14 in organic remains, scientists can estimate the time of death.
- Limitations of Carbon Dating:
- Carbon dating is not suitable for determining the age of non-living materials like rocks.
- After approximately 40,000-50,000 years, the amount of C-14 remaining becomes too small to detect accurately.
Radiometric Dating: Expanding the Dating Techniques
- Radiometric Dating Methods:
- Radiometric dating utilizes the decay of other radioactive elements found in non-living materials.
- These methods can estimate the age of very old objects due to the long half-lives of the radioactive isotopes involved.
- Potassium-Argon Dating:
- This method measures the decay of potassium into argon in rocks.
- By analyzing the ratio of these elements, scientists can determine the age of the rocks.
- Uranium-Thorium-Lead Dating:
- Uranium and thorium isotopes decay into stable lead isotopes.
- The ratios of these elements in a sample can provide insights into its age.
Cosmogenic Nuclide Dating: Uncovering Exposure to Sunlight
- Determining Exposure Time:
- Cosmogenic nuclide dating is used to determine how long an object has been exposed to sunlight.
- It relies on radioactive decays and is particularly useful for studying buried objects or changes in topology.
- Application to Ice Core Dating:
- Cosmogenic nuclide dating, also known as CRN, is commonly used to study the age of ice cores in polar regions.
- By analyzing the decay of certain isotopes, scientists can estimate how long the ice core has remained exposed.
Important Points:
- Carbon dating 📆:
- Determines the age of organic materials 🌱🔬
- Relies on the radioactive decay of Carbon-14 (C-14) 🧪☢️
- Measures the changing ratio of C-12 to C-14 to estimate time of death or origin ⏳🌿
- Limitations of carbon dating ❌:
- Ineffective for dating non-living objects like rocks 🚫🗿
- Not reliable for samples older than 40,000-50,000 years due to low C-14 levels ⌛️🔬
- Radiometric dating methods 🌍:
- Utilizes decay of other radioactive elements in non-living materials 🌋☢️
- Potassium-argon dating: Measures decay of potassium into argon in rocks 🗿⚛️
- Uranium-thorium-lead dating: Analyzes ratios of uranium, thorium, and lead isotopes 🌑🔬
- Cosmogenic nuclide dating (CRN) ☀️:
- Determines exposure time of objects to sunlight ☀️⏳
- Useful for studying buried objects or changes in topology 🌄🔬
- Frequently applied to dating ice cores in polar regions 🧊❄️
Why In News
The Allahabad High Court’s directive for a “scientific survey,” which encompasses carbon dating, has been issued to examine a “Shivling” reportedly discovered at the Gyanvapi mosque complex in Varanasi. This court order aims to leverage scientific methods, such as carbon dating, to shed light on the historical and archaeological significance of the found artifact.
MCQs about Carbon Dating and Radiometric Dating Methods
-
What is the primary basis of carbon dating?
A. Measurement of C-14 levels in living organisms
B. Comparison of C-12 to C-14 ratios in organic remains
C. Analysis of the decay of C-12 in atmospheric samples
D. Estimation of the half-life of C-14 in rocks
-
Which of the following is a limitation of carbon dating?
A. Inability to determine the age of rocks
B. Accuracy for samples older than 40,000-50,000 years
C. Dependence on the decay of C-12 isotopes
D. Application only to living organisms
-
Which radiometric dating method involves the decay of potassium into argon?
A. Uranium-thorium-lead dating
B. Carbon dating
C. Cosmogenic nuclide dating
D. Potassium-argon dating
-
What does cosmogenic nuclide dating primarily determine?
A. Exposure time of objects to sunlight
B. Decay rate of C-14 in organic remains
C. Age of ice cores in polar regions
D. Ratio of C-12 to C-14 in the atmosphere
Boost up your confidence by appearing our Weekly Current Affairs Multiple Choice Questions
![]()


