Daily Current Affairs : 25-November-2023
In a historic development, the UK drug regulator has granted approval for Casgevy, a gene therapy designed to cure sickle cell disease and thalassaemia. This revolutionary breakthrough employs the Nobel Prize-winning Crispr-Cas9 gene editing technology and marks a significant milestone in the field of medical science.
Key Details:
- Casgevy is the world’s first licensed therapy utilizing Crispr-Cas9 for gene editing, a technology acknowledged with the Nobel Prize in 2020.
- The therapy targets and edits the defective gene responsible for sickle cell disease and thalassaemia, potentially offering a lifelong cure.
- Traditional treatments for these blood disorders often require a bone marrow transplant, a procedure contingent on finding a closely matched donor.
- Casgevy, however, is a one-time treatment involving the extraction of blood stem cells through apheresis, a process used to filter various blood components.
How Does the Therapy Work?:
- Sickle cell disease and thalassaemia result from errors in the gene for haemoglobin, the protein responsible for oxygen transport in red blood cells.
- Casgevy utilizes the patient’s own blood stem cells, precisely edited with Crispr-Cas9 technology.
- The therapy targets the BCL11A gene, crucial for the transition from foetal to adult haemoglobin.
- By enhancing the production of foetal haemoglobin, which lacks the abnormalities associated with adult haemoglobin, Casgevy alleviates symptoms in both conditions.
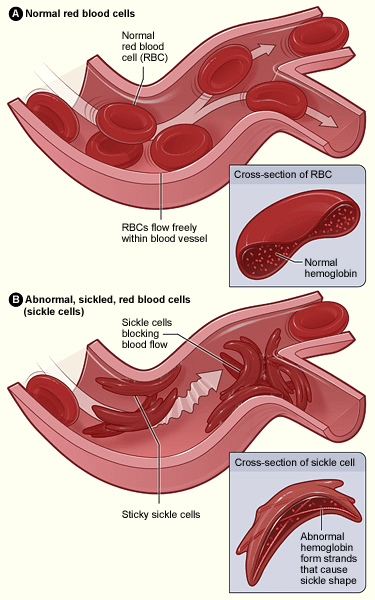
Sickle Cell Disease:
- Genetic errors cause red blood cells to assume a crescent shape, impeding their movement through vessels and leading to blocked blood flow.
- Symptoms include severe pain episodes, life-threatening infections, anaemia, and stroke.
- Manifestation occurs in individuals inheriting damaged genes from both parents, while carriers of a single gene lead normal lives.
Thalassaemia:
- Similar to thalassaemia, individuals inheriting gene pairs from both parents experience severe anaemia, leading to fatigue, shortness of breath, and irregular heartbeats.
- Blood transfusions are a lifelong necessity, resulting in excess iron accumulation, requiring chelation.
- India harbors the largest number of children with thalassaemia major globally, approximately 1-1.5 lakh.
Challenges of the Treatment:
- Cost: The therapy’s price, yet to be announced, is expected to be substantial, potentially reaching up to $2 million per patient.
- Absence of Local Manufacturing: Lack of local manufacturing facilities necessitates the transportation of harvested blood stem cells across countries.
Important Points:
- Casgevy Gene Therapy Approval:
- Groundbreaking approval by the UK drug regulator for Casgevy, a gene therapy targeting sickle cell disease and thalassaemia.
- Utilizes Crispr-Cas9 gene editing technology, awarded the Nobel Prize in 2020.
- Key Details:
- First licensed Crispr-Cas9 therapy globally, offering potential lifelong cure.
- Casgevy edits the faulty gene causing blood disorders, eliminating the need for closely matched bone marrow donors.
- One-time treatment involves apheresis to collect blood stem cells from the bone marrow.
- Mechanism of Action:
- Targets errors in the gene for haemoglobin, the protein in red blood cells carrying oxygen.
- Uses patient’s blood stem cells edited with Crispr-Cas9 to enhance production of foetal haemoglobin.
- Foetal haemoglobin lacks abnormalities found in adult haemoglobin, alleviating symptoms.
- Sickle Cell Disease:
- Genetic error leads to crescent-shaped red blood cells, causing blocked blood flow.
- Symptoms include severe pain, infections, anaemia, and stroke.
- Manifests in individuals inheriting damaged genes from both parents.
- Thalassaemia:
- Inherited gene pairs from both parents result in severe anaemia.
- Symptoms include fatigue, shortness of breath, irregular heartbeats.
- Lifelong blood transfusions and iron chelation required.
- India has the largest population of children with thalassaemia major globally.
- Challenges of the Treatment:
- Cost: Estimated to be high, potentially reaching $2 million per patient.
- Absence of Local Manufacturing: Harvested blood stem cells must be transported across countries due to the lack of local manufacturing facilities.
Why In News
The UK drug regulator recently approved a groundbreaking gene therapy for the cure of sickle cell disease and thalassaemia, marking a significant milestone in the advancement of medical science and offering renewed hope to patients worldwide.
MCQs about Casgevy Gene Therapy
-
What is Casgevy?
A. Traditional blood transfusion method
B. Nobel Prize-winning gene editing technology
C. Bone marrow transplant procedure
D. One-time treatment for diabetes
-
Which gene does Casgevy target for editing in sickle cell disease and thalassaemia?
A. BCL11A
B. FOXP2
C. BRCA1
D. HER2
-
What is the primary symptom of sickle cell disease?
A. Fatigue
B. Severe pain episodes
C. Irregular heartbeats
D. Shortness of breath
-
What is a significant challenge associated with Casgevy gene therapy?
A. Low treatment efficacy
B. Lack of global approval
C. Estimated high cost
D. Excessive side effects
Boost up your confidence by appearing our Weekly Current Affairs Multiple Choice Questions
![]()


