The Clinical Trials Registry of India (CTRI) is an online platform that allows for the registration of clinical trials being conducted in India. The platform was launched in July 2007 for use on a voluntary basis and is hosted with the Indian Council of Medical Research’s National Institute of Medical Statistics.
However, in June 2009, the Drug Controller General of India (DCGI) required that all trials be registered here. This was to ensure transparency and accountability for any study that involves human participants and investigates drugs, surgical procedures, preventative measures, lifestyle changes, medical devices, educational and behavioural treatments, or rehabilitation strategies.
Registration Process and Recognition as a Registry
The trial sponsor is required to publicly declare the trial, specify the investigators involved, establish criteria for selecting participants, obtain approval from the Drug Controller, and ensure that the ethics committees at each trial site also grant approval. The Clinical Trials Registry-India (CTRI) is a part of the International Clinical Trials Registry Portal and is acknowledged as the primary registry by the World Health Organisation.
Issues with the Indian Clinical Trials Registry
- Inconsistent enrollment records found in the Clinical Trials Registry of India (CTRI)
- Only 46% of trials were updated after the final enrollment
- CTRI offers a blank text box for information entry, resulting in the registry containing more than 1,000 unconventional categories
- Many trials do not provide required information as it is marked “optional” by CTRI
- Trials have internal inconsistencies, such as filling in the wrong type of trial due to confusion regarding definitions
- Non-standardized information about cities causes confusion and repetition in the registry
- Variation in the name of the principal investigator provided, wrong spelling, use of abbreviations, or different surnames hinder identifying important individuals
- CTRI allows for primary sponsors to be classified under several categories, but no specific definition is provided for such categories
- Unclear data leads to overlap and confusion
- Wrong data about whether a trial is registered prospectively or retrospectively can be classified as misleading information
Possible Solutions
- Making registration of clinical trials on CTRI compulsory and enforcing compliance by the Central Drugs Standard Control Organisation (CDSCO)
- Ensuring compliance with WHO regulations for primary registration
- Improving the information provided with each record on CTRI, including information regarding the audit trail and a ‘results’ section in the register
- Executing a strategy for sharing data and exceeding WHO standards for clinical trial registration
- Increasing transparency by bringing all documentation on every trial to one platform and providing public access to regulatory documents through CDSCO
- Withholding approval for new applications until correct data is submitted and correcting older records to ensure accurate record-keeping
- Establishing CTRI as a permanent activity and hiring staff on a five-year contract, as it is currently a temporary activity of ICMR with staff serving for only 15 years.
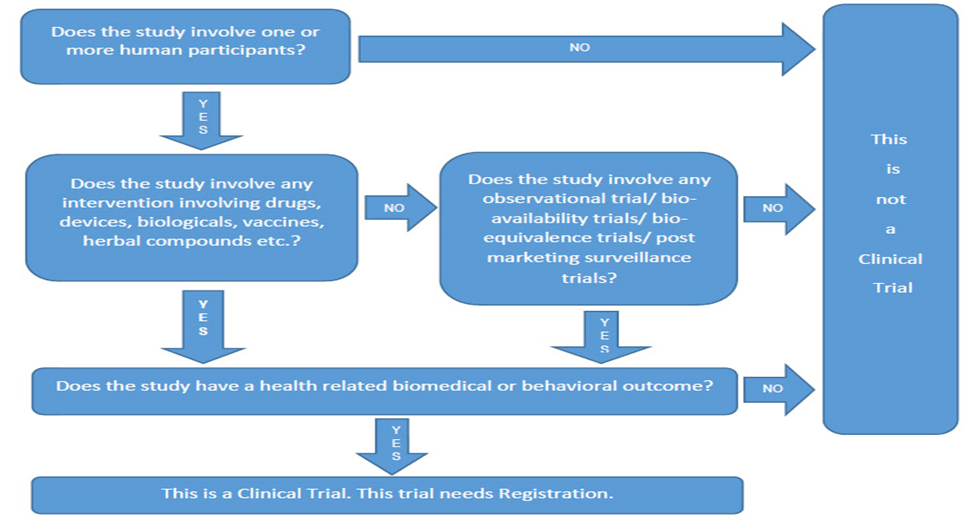
Why In News
The Clinical Trials Registry of India is a vital resource for tracking clinical trials being conducted in India. However, the platform has been encountering several problems, including inadequate data quality, incomplete information, and low registration rates. In this article, we will explore these issues in detail and offer potential solutions to improve the functioning of the Clinical Trials Registry of India.
MCQs about Clinical Trials Registry of India
-
What is the Clinical Trials Registry of India?
A. A physical location in India where clinical trials are conducted.
B. An online platform for registering clinical trials conducted in India.
C. A government agency responsible for overseeing clinical trials in India.
D. A non-profit organization providing medical care to underprivileged communities in India.
-
What are some issues currently facing the Clinical Trials Registry of India?
A. Insufficient funding for clinical trials.
B. Inadequate technology infrastructure.
C. Lack of awareness among researchers about the registry.
D. Both B and C.
-
Why is it important to have a registry for clinical trials?
A. It helps researchers keep track of their own studies.
B. It allows for transparency in the clinical trial process.
C. It ensures that only the most qualified participants are selected for trials.
D. It helps to reduce the cost of conducting clinical trials.
-
What is the purpose of the Indian Council of Medical Research?
A. To provide medical care to underprivileged communities in India.
B. To conduct clinical trials in India.
C. To oversee and regulate medical research in India.
D. To promote the use of traditional medicine in India.
Boost up your confidence by appearing our Weekly Current Affairs Multiple Choice Questions
![]()


