Daily Current Affairs : 2-November-2023
In a groundbreaking development, the Central Drugs Standard Control Organisation (CDSCO) has granted market authorization for NexCAR19, India’s first indigenously-developed CAR-T cell therapy, to ImmunoACT, a company incubated by IIT Bombay. This significant achievement marks a turning point in the field of cancer treatment in India and has captured widespread attention.
Understanding CAR-T Cell Therapy: A Game-Changer in Cancer Treatment
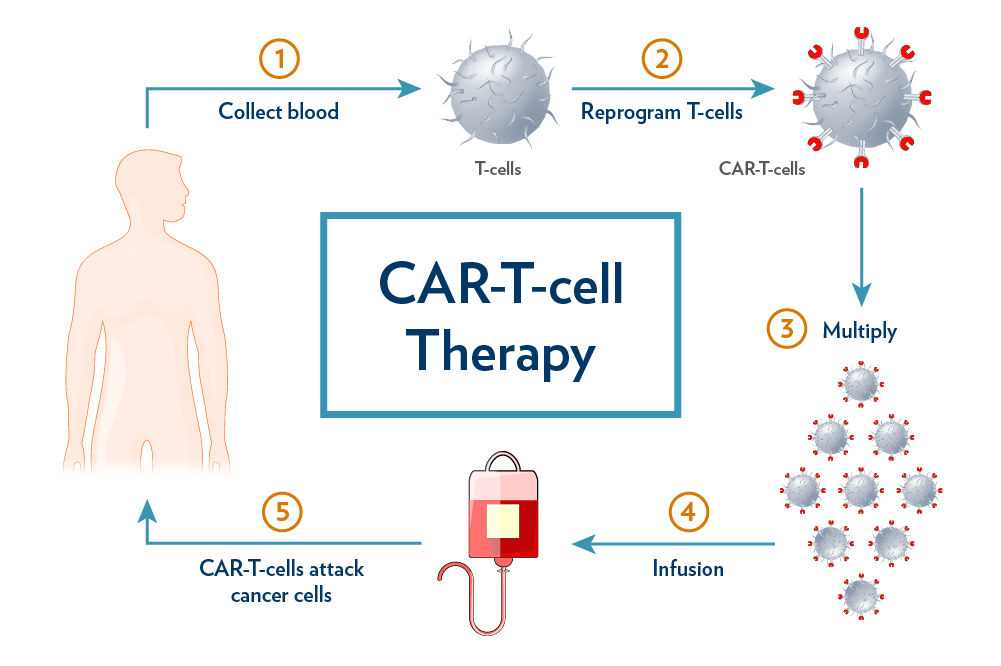
CAR-T cell therapy, short for Chimeric Antigen Receptor T-cell therapy, represents a revolutionary approach to treating cancer. Unlike traditional chemotherapy and immunotherapy, CAR-T therapy modifies T-cells, turning them into potent cancer fighters that are genetically engineered to target and eliminate cancer cells. This innovative therapy holds the promise of not just prolonging life but potentially curing cancer, offering a lifeline for patients who have exhausted other treatment options.
NexCAR19: India’s Indigenous CAR-T Therapy
NexCAR19, a product of cutting-edge research in India, targets cancer cells carrying the CD19 protein, a marker for specific types of blood cancers. This therapy allows CAR-T cells to recognize and attach themselves to cancer cells, initiating the process of elimination. Remarkably, India now stands among the pioneering developing nations with its own indigenous CAR-T and gene therapy platform, a feat previously reliant on imports from the United States or Europe.
Who Can Benefit from NexCAR19?
NexCAR19 offers hope for patients with B-cell lymphomas who have not responded to standard treatments like chemotherapy. The procedure involves extracting the patient’s blood, genetically modifying the T-cells, and then reintroducing them into the patient’s body. Recovery typically occurs within two weeks after one cycle of treatment, with a significant percentage of patients responding positively to the therapy, achieving complete remission.
Safety and Accessibility: Addressing Concerns
While NexCAR19 boasts significantly lower drug-related toxicities and minimal damage to neurons, concerns about accessibility and affordability persist. Currently priced between Rs 30-40 lakh, the therapy may not be accessible to everyone. The hope is to reduce costs to Rs 10-20 lakh as technology advances and manufacturing processes improve. Additionally, the extent of insurance coverage for this expensive treatment remains uncertain, posing challenges for widespread accessibility.
Important Points:
- CAR-T Cell Therapy:
- Revolutionary approach modifying T-cells into potent cancer fighters.
- Genetically engineered T-cells target and eliminate cancer cells.
- Potential for curing cancer, offering hope for patients with limited treatment options.
- NexCAR19:
- Developed in India, targets cancer cells carrying CD19 protein.
- Allows CAR-T cells to recognize and eliminate cancer cells.
- India joins developed nations with its indigenous CAR-T and gene therapy platform.
- Patient Eligibility and Procedure:
- Suitable for B-cell lymphoma patients unresponsive to standard treatments like chemotherapy.
- Blood extracted, T-cells genetically modified, and reintroduced into the patient.
- Recovery typically occurs within two weeks after one treatment cycle.
- Safety and Accessibility:
- NexCAR19 leads to lower drug-related toxicities and minimal damage to neurons.
- Current cost: Rs 30-40 lakh; efforts to reduce it to Rs 10-20 lakh ongoing.
- Concerns about insurance coverage and accessibility for all patients remain.
Why In News
The Central Drugs Standard Control Organisation (CDSCO) granted market authorisation for NexCAR19, India’s first indigenously-developed CAR-T cell therapy, to ImmunoACT, a company incubated by IIT Bombay, marking a significant milestone in the country’s biotechnology landscape and paving the way for groundbreaking advancements in cancer treatment.
MCQs about NexCAR19 CAR-T Therapy Pioneers Cancer Treatment Landscape
-
What is NexCAR19?
A. A chemotherapy drug
B. India’s first indigenously-developed CAR-T cell therapy
C. An immunotherapy vaccine
D. A standard treatment for B-cell lymphomas
-
What is the primary function of CAR-T cell therapy?
A. To manage cancer symptoms
B. To prolong a patient’s life by a few months
C. To cure cancer and provide lifelong benefits
D. To prevent cancer from recurring
-
Who is eligible for NexCAR19 therapy?
A. Patients of all age groups
B. Only children with B-cell lymphomas
C. Patients aged 15 years and older with B-cell lymphomas unresponsive to standard treatments
D. Patients with any type of cancer
Boost up your confidence by appearing our Weekly Current Affairs Multiple Choice Questions
![]()


